(Solved) : 55 Mass Hci Would Need Added 250 Ml Solution Containing 0500 M Nac Hso2 0500m Hc2hso2 Make Q32431373 . . .
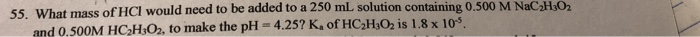 55. What mass of HCI would need to be added to a 250 mL solution containing 0.500 M NaC HsO2 and 0.500M HC2HsO2, to make the pH 4.25? Ka of HC2HsO2 is 1.8 x 10 Show transcribed image text
55. What mass of HCI would need to be added to a 250 mL solution containing 0.500 M NaC HsO2 and 0.500M HC2HsO2, to make the pH 4.25? Ka of HC2HsO2 is 1.8 x 10 Show transcribed image text
Expert Answer
Answer to 55 Mass Hci Would Need Added 250 Ml Solution Containing 0500 M Nac Hso2 0500m Hc2hso2 Make Q32431373 . . .
OR